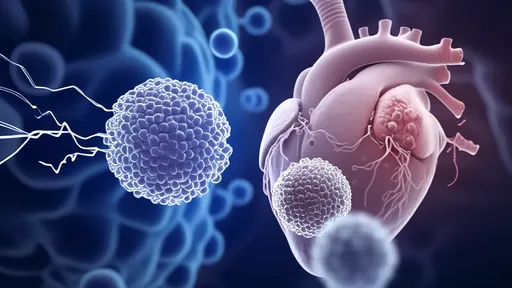
In a groundbreaking development in cardiac regenerative medicine, researchers have made significant strides in using engineered heart tissue patches to repair damaged areas following myocardial infarction. These so-called "cardiac patches," composed of layers of functional cardiomyocytes, offer new hope for restoring heart function where traditional treatments fall short. The technology represents a paradigm shift from symptom management to actual tissue regeneration.
The concept of cardiac patches stems from the heart's limited innate capacity for self-repair. Unlike some organs that can regenerate after injury, the human heart forms scar tissue after ischemic events, leading to permanent functional impairment. Scientists have spent decades attempting to overcome this biological limitation through various approaches, with cell sheet technology emerging as one of the most promising solutions in recent years.
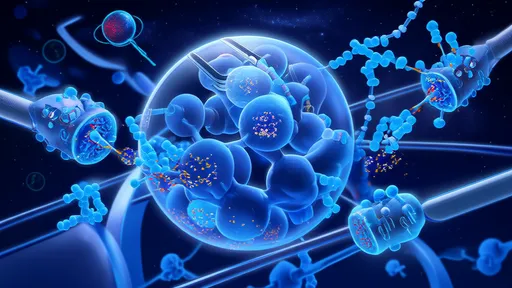
At the core of this innovation lies the ability to grow living, beating heart cells in thin, coherent layers that can be surgically applied to infarcted areas. These myocardial sheets typically measure just a few cell layers thick – thin enough to allow nutrient diffusion yet substantial enough to provide mechanical support and electrical integration with the host tissue. The patches are created by culturing cardiomyocytes derived from various sources, including induced pluripotent stem cells (iPSCs), on temperature-responsive surfaces that allow for easy detachment of intact cell sheets.
Recent preclinical studies have demonstrated remarkable outcomes. When applied to damaged hearts in animal models, the patches not only survived transplantation but gradually integrated with the host myocardium. Perhaps most impressively, researchers observed improvements in cardiac output, reduced scar size, and restoration of electrical conduction across previously non-functional areas. The living patches appear to secrete beneficial growth factors while simultaneously providing structural support to the weakened heart wall.
One of the critical challenges has been establishing proper vascularization of the implanted tissue. Without adequate blood supply, the patches risk necrosis after transplantation. Innovative solutions have emerged, including pre-vascularizing the patches by incorporating endothelial cells during the culturing process or creating microchannel networks within the engineered tissue. Some research teams have experimented with timed release of angiogenic factors to encourage host blood vessels to grow into the graft.
The electrical integration of the patch with the surrounding myocardium presents another complex hurdle. Cardiomyocytes must not only beat in synchrony but also establish proper gap junctions with host cells to prevent arrhythmias. Advanced tissue engineering approaches now incorporate conductive nanomaterials or specific alignment techniques to ensure proper electrical propagation across the graft-host interface.
Clinical translation of this technology has begun cautiously, with several human trials underway in Japan and Europe. Early-phase studies focus on safety and feasibility, using autologous cell sources to minimize immune rejection. While results appear promising, researchers emphasize that significant questions remain regarding optimal cell sources, patch thickness, delivery methods, and long-term outcomes. The field must also address scalability issues and the high costs associated with personalized cell therapies.
Looking ahead, scientists envision combining cardiac patches with other regenerative approaches such as gene editing or biomaterial scaffolds to create even more sophisticated repair systems. Some laboratories are working on "smart patches" that could respond to mechanical stress or release therapeutic compounds on demand. Others are exploring minimally invasive delivery methods that wouldn't require open-heart surgery.
The potential impact of successful cardiac patch technology cannot be overstated. Heart disease remains the leading cause of death worldwide, with millions suffering from heart failure following myocardial infarction. If current research trajectories hold, these living myocardial patches may transform cardiology practice within the coming decade, offering a genuine regenerative option where only palliative care exists today.
Ethical considerations and regulatory pathways will play crucial roles in how quickly this technology reaches patients. The use of stem cells, particularly embryonic sources, continues to spark debate, though the field has largely shifted toward iPSC-derived alternatives. Regulatory agencies face the challenge of balancing accelerated approval for life-saving treatments with thorough evaluation of long-term risks in what is essentially a dynamic, living therapy.
As research progresses, the cardiac patch approach may expand beyond post-infarction repair to other cardiac conditions. Investigators are already exploring applications for congenital heart defects, right ventricular failure, and even as a platform for drug testing. The convergence of tissue engineering, stem cell biology, and materials science in this field exemplifies the potential of interdisciplinary approaches to solve complex medical challenges.
The journey from petri dish to patient has been long and will likely continue to present obstacles, but the cardiac patch story represents one of the most tangible examples of regenerative medicine's potential. With each technological refinement and clinical milestone, the vision of truly healing damaged hearts moves closer to reality, promising to rewrite the prognosis for millions of cardiovascular patients worldwide.

By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025