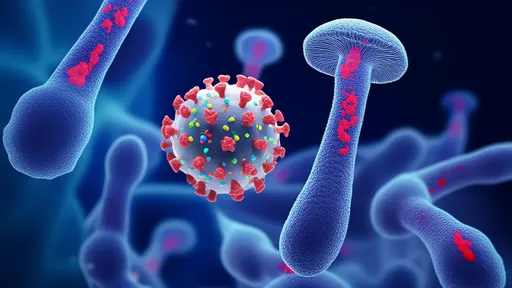
In the microscopic battleground where viruses invade host cells, a remarkable molecular machinery operates with precision that rivals human-engineered systems. Recent breakthroughs in structural biology have revealed how certain viruses employ spring-loaded fusion proteins to breach cellular defenses—a discovery that reshapes our understanding of infection mechanics and opens new avenues for therapeutic intervention.
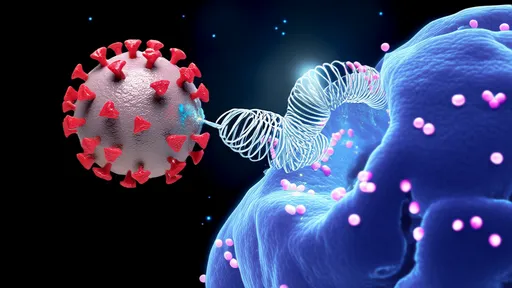
The viral fusion protein acts as nature’s microscopic lockpick. Like a coiled spring released upon contact, these proteins undergo dramatic conformational changes when encountering specific receptors on host cells. Researchers now compare this mechanism to a "molecular mousetrap," where energy stored in metastable protein structures is harnessed to force membrane fusion. This process, occurring at nanometer scales, allows viral genetic material to infiltrate the cytoplasm with terrifying efficiency.
High-resolution cryo-EM studies have captured these fusion proteins in action. The pre-fusion state shows tightly packed helical bundles resembling compressed springs, while post-fusion structures reveal completely extended conformations. What makes these proteins extraordinary is their dual functionality—they not only recognize host cells but also contain the mechanical force required for membrane penetration. This economy of design has inspired biomimetic engineers seeking to develop targeted drug delivery systems.
Temperature plays a crucial role in the spring-loaded mechanism. Many fusion proteins remain stable at physiological temperatures but become triggered by subtle pH changes in cellular compartments. This explains why some viruses can remain dormant outside hosts yet spring into action upon entering endosomes. The precision of this environmental sensing suggests evolutionary refinement over millions of years, resulting in what virologists now describe as "perfectly tuned biological springs."
Beyond virology, these discoveries impact vaccine design. Stabilizing fusion proteins in their pre-fusion conformation has become the holy grail for developing vaccines against RSV, HIV, and coronaviruses. The ability to "trap the spring" before its release allows immune systems to recognize and neutralize viruses more effectively. This approach underpins several next-generation vaccine platforms currently in clinical trials.
Structural biologists emphasize that we’re witnessing a paradigm shift in membrane fusion understanding. The spring-loaded model replaces older "sticky patch" theories, providing clearer explanations for how viruses overcome the substantial energy barriers of membrane fusion. This knowledge is already being applied to develop fusion inhibitors that could block viral entry by preventing the spring mechanism from releasing.
As research continues, scientists are discovering surprising variations of this mechanism across different virus families. From the harpoon-like fusion spikes of Ebola to the jackknife motions of influenza proteins, nature has evolved multiple solutions to the membrane fusion challenge. Each discovery provides new pieces to the puzzle of viral infection—and new targets for antiviral strategies in an age of emerging pathogens.

By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025