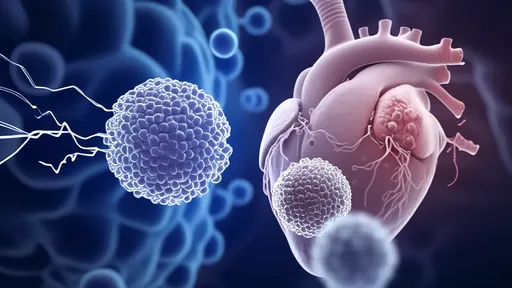
In a groundbreaking development that could revolutionize the treatment of heart failure, scientists are exploring the potential of mitochondrial transplantation to rejuvenate failing hearts. The concept, often referred to as "mitochondrial borrowing," involves transferring healthy mitochondria from donor cells to damaged heart tissue, effectively "rebooting" the organ's energy production. This innovative approach challenges conventional cardiology paradigms and offers hope for patients with end-stage heart disease.
The human heart is a metabolic powerhouse, consuming vast amounts of energy to maintain its relentless pumping action. Mitochondria, often called the "powerhouses of the cell," generate about 90% of the chemical energy needed for survival. When these tiny organelles malfunction due to disease or aging, the consequences can be catastrophic, particularly for an energy-demanding organ like the heart. Traditional treatments for heart failure focus on managing symptoms rather than addressing the underlying cellular energy deficit—a gap that mitochondrial transplantation aims to fill.
Researchers at several leading institutions have demonstrated remarkable success in animal models. In one notable study, scientists extracted mitochondria from the skeletal muscles of healthy subjects and injected them directly into oxygen-deprived heart tissue. The transplanted mitochondria were rapidly taken up by heart cells, integrating seamlessly into their new environment. Within hours, the treated areas showed significant improvements in energy production and contractile function, while untreated regions continued to deteriorate.
The procedure's mechanism remains partially mysterious, but evidence suggests the transplanted mitochondria don't permanently replace damaged ones. Instead, they appear to provide a temporary "energy boost" that allows stressed heart cells to repair their own mitochondrial networks. This phenomenon resembles jump-starting a car battery—the external power source enables the system to regain self-sufficiency. Some researchers speculate that the transplanted mitochondria may also release signaling molecules that activate cellular repair pathways.
Clinical applications are already underway, albeit cautiously. Boston Children's Hospital has pioneered first-in-human trials for pediatric patients with ischemia-reperfusion injury following cardiac surgery. Early results show promise, with treated patients demonstrating faster recovery of heart function compared to historical controls. Meanwhile, other research teams are exploring variations of the technique, including using mitochondria derived from stem cells or modifying donor mitochondria to enhance their therapeutic potential.
Despite the excitement, significant challenges remain. The logistics of mitochondrial isolation and transplantation require precise timing—the organelles begin losing function quickly after removal from their host cells. Researchers are experimenting with various preservation techniques, including special nutrient solutions and temperature controls, to extend mitochondria's viability outside the body. There are also unanswered questions about optimal dosing, delivery methods, and potential immune reactions to the transplanted organelles.
The implications extend beyond cardiology. If mitochondrial transplantation proves consistently effective, it could transform treatment for other energy-intensive organs affected by mitochondrial dysfunction, including the brain in neurodegenerative diseases and skeletal muscles in certain myopathies. Some scientists envision a future where "mitochondrial banks" store these organelles for therapeutic use, similar to blood banks today.
Ethical considerations accompany these scientific advances. The most efficient mitochondrial sources currently come from healthy muscle biopsies, raising questions about donor availability and consent. Researchers are actively investigating alternative sources, including laboratory-grown mitochondria and potentially even synthetic analogs. Regulatory frameworks will need to evolve to address these novel biological therapies that don't fit neatly into existing categories of drugs or transplants.
As the field progresses, collaboration between cardiologists, cell biologists, and bioengineers is intensifying. Annual conferences now feature dedicated sessions on mitochondrial medicine, and venture capital has begun flowing into startups exploring commercial applications. While experts caution that widespread clinical use may still be years away, the pace of discovery suggests that mitochondrial therapy could become a standard weapon in medicine's arsenal against heart failure sooner than anticipated.
The story of mitochondrial transplantation exemplifies how revisiting fundamental biological concepts can yield unexpected clinical breakthroughs. By focusing on cellular energetics—an aspect often overlooked in organ-level treatments—researchers may have found a way to accomplish what drugs and devices cannot: directly restoring the heart's vital spark. As one leading researcher poetically remarked, "We're not just treating disease; we're reigniting the flame of life itself."

By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025
By /Jul 14, 2025